Introduction: Recently discovered Micro RNAs (miRNA) are small, single stranded non-coding RNAs, which bind to target mRNA and are involved by translational repression and mRNA degradation. These newly found miRNA are a potentially useful and fresh perspective into gene expression and regulation. Abnormal miRNA activity/expression has recently been correlated with a number of gynecological conditions in humans; such as: malignancies and infertility disorders.
The authors identified several miRNA expression profiles from the eutopic and ectopic endometrium of women with endometriosis and identified a novel miRNA which has been associated with a molecular pathway which is likely to be important in the pathophysiology of endometriosis.
This paper reviews the consensus on miRNAs, their biogenesis, function and regulation and then examines miRNA expression in female endometrial pathologies and outlines how further study of endometrial miRNAs could possibly have practical applications of diagnosis and therapy of endometriosis.
Methods: The authors undertook and overview of the basic miRNA biology based on the recent and seminal published research in this field. They then conducted a systematic review of the literature on miRNAs in their relation to the mammalian female reproductive tract with emphasis on the endometrium and endometriosis.
Discovery and Mechanisms of miRNA’s:
miRNA’s were first discovered in 1993, but has only been since the year 2000 that their importance, function and structure has been a focus of research. Researchers use high throughput methods to locate and identify miRNAs in order to study their role in diseases. Micro-arrays using miRNA gene probes are used in conjunction with genome wide and in situ miRNA detection techniques to study and locate miRNAs.
Studying the function of miRNAs involves in silico algorithms that were recently developed and used to predict the target mRNA of a given miRNA. They function by searching for mRNAs that show a homology to the miRNA in the 5’ ‘seed sequence’. Multiple algorithms are used in order to ensure accuracy and precision when predicting mRNA targets. Experimental procedures are then used to validate the in silico analysis of mRNA targets and miRNA repression effects, these procedures include but are not limited to: qRT-PCR and Western blots combined with a luciferase reporter assay.
It is necessary to understand a few key points when analyzing miRNA data, when an miRNA is up-regulated (abundant in the cell) its target mRNA is repressed and vice versa. Analysis is therefore conducted on the effect of cellular mRNA levels and their subsequent cellular protein profiles after manipulation of their corresponding miRNAs. In vivo research concerning miRNAs and disease is mainly conducted using a Mus musculus (mouse) model and various genetic modifications.
Current model of miRNA expression and Processing:
The current literature indicates that mammalian miRNA genes are in embedded introns of protein coding genes. The miRNAs are transcribed in the cell’s nucleus by RNA polymerase II and after post- transcriptional modifications pri-miRNAs leave the nucleus as hairpin structures about 71 nt in length. The pri-miRNA is further processed in the cytoplasm into short miRNA duplexes, the antisense strand is left to degrade and the mature miRNA is ready to act upon its target mRNA.
Model of miRNA mediated gene repression:
The miRNA binds to multiple sites in the 3’ untranslated region of the target mRNA through imperfect base pairing. Depending on the strength of the complementary base pairing, the mRNA can undergo decay or translational inhibition or in the extreme cases, endonucleotic cleavage and mRNA degradation can occur. Repressed mRNAs aggregate in cytoplasmic p-bodies and can become active again under cellular stress. miRNA families can act together to generate amplified repression on specific cellular functions when these regulatory mechanisms malfunction detrimental effects can occur.
miRNAs in endometriotic lesion development:
As mentioned in a previous blog, Endometriosis is the presence of endometrial glands and stroma in tissues outside the uterus. ~ 6-10% of women suffer from painful menstruations, and chronic pelvic pain due to endometriosis. Microarrays of endometrial animal models have identified endometriosis-associated transcripts that encode proteins in pathways that mediate inflammation, tissue remodeling, apoptosis, cellular proliferation and angiogenesis. It has been found that many mRNA transcripts are differentially regulated in endometriotic lesions compared to normal endometrial tissues. In silico analysis of expression levels between mRNAs present and proteins in endometriotic lesions indicate that there is some evidence that miRNA gene regulation could be occurring in this abnormal endometrial tissue.
Two recent studies conducted by Pan et al. and Ohlsson Teague et al. identified 48 and 22 miRNAs (respectively) that were only expressed in women diagnosed with endometriosis. The differences in findings can be attributed to the studies using tissue samples from different stages in the menstrual cycle and different stages of endometriosis. These studies indicate that these identified miRNAs are differentially expressed throughout the menstrual cycle in women with endometriosis. Only 8 miRNAs were expressed in both studies and these miRNAs were not in concordance in their direction of mis-regulation between the two studies. This is probably due to the diverse methods each group used to conduct the study.
The authors of this review (Ohlsson Teague et al. ) presented models of theoretical miRNA interactions causing endometriotic lesions based on the functions of experimentally confirmed mRNA targets of miRNAs associated with endometriosis. The authors suggest seven conditions of endometrial lesions are controlled by miRNA regulation:
Hypoxia: miRNAs: 20, 200b, 15b and 16 are repressed by hypoxic conditions. These repressions in turn cause transcription of two pro-angiogenesis proteins which promotes the growth of endometrial deposits outside of the uterus.
Inflammation: Pro-inflammatory conditions in endometriotic lesions cause an increase in transcription of COX-2, a rate-limiting enzyme in the pro-inflammatory prostaglandin pathway, which has an angiogenesis promoting effect. miR-199a and miR-16 suppress COX-2 and decreases prostaglandin production in normal tissues. In ectopic endometrial tissues it was found that the levels of these two miRNAs were reduced resulting in an increased production of prostaglandins, cell proliferation and angiogenesis.
Tissue Repair: TGF-β has been found to be up-regulated in women with endometriosis and is a central component in cellular growth signaling. This up regulation promotes endometrial tissue remodeling. TGF-β is shown to be regulated by miR-21 and miR-14, these two micro RNAs are shown to be down regulated in women with endometriosis. Conversely, two miRNAs (1 & 194) are up regulated in ectopic endometrial tissue. These two miRNAs act to suppress TGIF, which is the factor that acts to suppress TGF-β.
Cell Growth, proliferation and apoptosis: BCL-2 mediates apoptotic resistance in endometrial cells, miR-15b and 16 target BCl-2 to reduce the cell’s resistance to apoptosis. Therefore reduced miR-15b and 16 levels in endometrial tissues lead to an increases resistance to apoptosis. Cell proliferation is promoted by a cell cycle regulator which is suppressed by miR-126 and miR-145, in ectopic endometrial tissues, these miRNAs are expressed in high levels causing a decrease in cell proliferation. Therefore the miRNAs that are down regulated enhance cell survival and those that are up regulated reduce cell proliferation.
Extracellular Matrix Remodeling: Endometriotic lesions have been associated with abnormal ECM proteins and it has been found that decreased levels of miR-129c have led to an increase in transcription of ECM proteins.
Angiogenesis: Vascularization is very important in endometriotic lesion development. miR-126 suppresses angiogenic pathways . This miRNA is found in low levels in ectopic endometrium therefore angiogenesis is active in endometriotic lesions.
MiRNAs and Malignant Transformation in Endometriosis:
Cancer Risk: Women with endometriosis have been correlated with a risk of ovarian cancer with ratio of endometriosis patients developing cancer over non –endometriotic patients between 1.3 and 2.2, suggesting that malignant changes can occur in ectopic endometrium of some women.
miRNA abnormalities in cancer:
The normal functioning of miRNAs are frequently in cancers due to the mechanism of the disease. These dysregulations have a variety of effects on miRNAs such as: promoting tumour growth by up regulating miRNAs that target tumour suppression. The authors suggest that the miRNA profiles of endometriosis may regulate transcripts involved in the cell cycle and cancer; they propose that miRNA activity allows the ectopic endometrium to be highly regenerative without proliferating uncontrollably thereby maintaining a benign and sustainable ectopic endometrial mass.
miRNAs in endometriosis and subfertility:
There is strong evidence that miRNAs are integral in subfertility but no studies have directly explored this topic. The authors present some evidence that miR-21 and 26a up regulation in women with endometriosis may alter or deter implantation of a fertilized embryo.
Theraputic and diagnostic potential of miRNAs in endometriosis:
Diagnosis of endometriosis is commonly 6-12 years after the first onset of symptoms, which leads to an increased severity of the disease. The authors propose using miRNAs as biomarkers for diagnosis of endometriosis due to their easy detectability in tissues from routine pathology archives, their relative resistance to RNase degredation and the improved accuracy over using mRNAs for pathology detection. The authors also postulate a serum blood test for miRNAs associated with endometriosis based upon a study showing that miRNAs associated with prostate cancer were found serum of mice.
miRNA as a theraputic target in endometriosis:
The authors have proposed a variety of mechanisms by which miRNAs could potentially be used to treat ectopic endometriosis which include:
antagonism of miRNAs- where synthetic oligonucleotide antagonists would base pair with miRNAs to prevent suppression of target mRNAs
miRNA decoys- where introduction of synthetic target sequences compete with endogenous mRNA for miRNA regulation, thereby relieve the suppression from the endogenous mRNA.
miRNA mimics- where in order to increase miRNA regulation, synthetic double stranded pre-miRNA duplexes become activated by the endogenous activation pathways and act a miRNA’s to suppress the target mRNAs.
At this time, there are many obstacles in using these mechanisms as endometrial therapy in humans due to difficulties in finding appropriate animal models, delivering the miRNAs to specific cells in vivo and the unknown possibility side effects.
Conclusions:
Recent studies into differential miRNA expression in endometriotic tissue has led to the creation of new bioinformatics approaches to study the functions of miRNAs linked to this disorder. It is already clear that miRNAs play a very important role in this disease and as research progresses this field offers the potential for improvements in diagnostic and treatment protocols.
My Opinion:
In my opinion, the concepts presented by this review were extremely interesting and the therapeutic possibilities for endometriosis are very exciting. However, this review was so packed full of information that I feel it should have been split into two papers: one focusing on miRNAs, their discovery and the laboratory techniques used in association with them and one focusing on miRNAs and their implications with endometriosis. In its present form the paper is too full of information for a reader to absorb and the authors were not fully able to explore the topics relating to endometriosis.
When reading the latter half of the review I found it very difficult because I got the sense that the authors could explain their ideas and concepts better if they just explored them a little further. I noticed that the paper had an excellent flow and I was grabbed by the exciting novel topic; however by the middle to the end of the paper, I found it increasingly difficult to follow and comprehend the point the authors were trying to make due to a jumbled writing style and a hesitance to make broad summarizing statements. The authors made the paper further jumbled by creating multiple subsections for each topic with many of them having little to do with the topic. The information was presented in a poorly organized manner that left me disappointed because the topic was very interesting; however the potential therapeutic and diagnostic possibilities outlined in the last sections of this review saved the topics presented in this review from being a complete disappointment.
More information can be found by reading the paper!
Ohlsson Teague, E. Maria C., Print, Cristin G. Print and Hull, M. Louise. (2009) The role of microRNAs in endometriosis and associated reproductive conditions. Human Reproductive Update:Vol 00, No.O pp. 1-24.
Tuesday, November 17, 2009
Tuesday, October 27, 2009
The Endometrium
The endometrial tissue (part of the uterine mucosa) is the innermost tissue layer that lines the upper vaginal cavity in placental females. In humans, the upper vaginal cavity is derived from the mesenchymal epithelial tissue of the Müllerian ducts.
The endometrium is triangular and continuous, in its upper corners, with the lumina of the fallopian tubes and the cervix at it lower point.
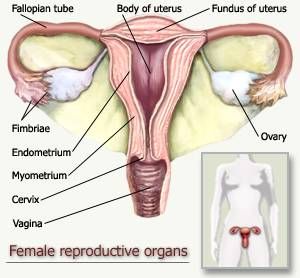
Figure 1: Diagram of the Female Reproductive Tract.
The endometrial lining is composed of columnar epithelial cells supported by a cellular stroma with tubular glands.

Figure 2: Endometrial Tissue Composed of Columnar Epithelia, Stroma and Tubluar Glands(Arrows).
The uterine mucosa is composed of the isthmus and corpus. The isthmus is thin mucosa and is not very responsive to hormonal stimuli; it is located between the endometrium and the endocervix in the lower portion of the uterine cavity. The corpus (endometrium) is a mucosa which is fully responsive and affected by female hormonal stumuli and their cyclic fluctuations. The corpus itself can be divided into two layers: the lower layer known as the basalis and the upper layer known as the functionalis.

Figure 3: Diagram of the Female Uterus in Order to Highlight the Isthmus and the Corpus of the Endometrium.
The basalis is the zone of relative inactivity adjacent to the myometrium of the uterus. The basalis is an area of weakly proliferative gland cells and dense spindle shaped stromal cells. It is known as the reserve cell layer and it is responsible for regenerating the endometrium after the functionalis has shed. Its thickness remains constant throughout the menstrual cycle. The functionalis is a region of proliferative glandular cells which are arranged in sheets due to the mutual adhesiveness between gland cells. Little proliferation of cells is shown in the early phases of the menstrual cycle; however as estrogen levels rise, proliferation is more apparent and in the late proliferatory phase, the epithelial layer changes to a pseudostratified or stratified columnar epithelium. Since the endometrial tissue is highly sensitive to female steroid hormone levels in the body, its composition undergoes many changes depending on a woman’s age and fertility.
Before Puberty: Estrogen and progesterone levels are low and the endometrium is atrophic showing a lack of mitotic activity in its gland it is similar to the endometrial state after menopause.
After Menopause: The endometrium is once again atrophic. The lining is thin and smooth. The glands are a single layer of flattened cuboidal cells and the stromal cells are spindled and closely packed.

Figure 4: Histological Section of an Atrophic Endometrium.
During the Reproductive Years: The endometrium is composed of:
• Glandular epithelia
• Proliferative glands
• Secretory glands
• Surface glandular epithelium
• Stoma
• Vasculature
The endometrial cells synchronously proliferate, differentiate and shed cyclically every 28 days in response to estradiol and progesterone changes in the female human body. This 28 day cycle is known as the Menstrual Cycle and is categorized into phases.
Proliferation Phase:
Days 1-14 of the cycle. This phase exhibits ovarian secreted, estradiol induced proliferation of stroma, gland cells and vasculature.
Days 1-4 mark the menstrual phase which will be discussed below.
Days 4-13: proliferation of the endometrial lining ensues. The functionalis tissues increase in thickness with an increase in gland cells, stroma and vasculature. The glands and blood vessels increase in size and number faster than the stroma, causing them to become coiled giving the arteries a characteristic spiral conformation. The stromal cells become enlarged by edema and an increase in glycogen content and pack closer together.
Secretory Phase:
Occurs after ovulation on day 14. Estradiol and progesterone secreted from the corpus luteum induce secretions and differentiation of stromal cells into predecidualized cells.
Days 14-28: secretions are noticed in the epithelial layer The endometrium is abundant with closely packed gland and enlarged glands. In late secretory phase, the stomal cells become predecidual and surround the spiral arteries. The secretions slow down and neutrophils and lymphocyte-like granulocytes infiltrate the stroma. These granulocytes are thought to release relaxin that dissolve reticular fibers found near the stromal cells just before endometrial shedding of the menstrual phase.
If implantation does not occur the menstrual phase progresses.
Menstrual Phase:
Days 1-4 Endometrial shedding takes place. Around the 28th day of the menstrual cycle, there is a sudden reduction in the thickness of the functionalis due to a decreased steroid hormone levels in the body. This causes impaired blood flow to the upper portion of the endometrium leading to ischemia, necrosis and hemorrhage of the endometrial lining. During this phase, there is an increase in leukocytes and fibrin thrombin appears in blood vessels in the area. The collapsed stroma, ruptured glands, neutrophils, granulocytes and ruptured vasculature is what is shed during this phase.

Figure 5: Diagram of Endometrial Thickness in Response to Hormonal Stimulation.
However if implantation does occur before shedding, there is a resurgence of gland secretions and exaggerated stromal decidualization where almost all endometrial stroma is converted to sheets of large decidual cells.
Cells of the Endometrium
-Ciliated glandular cells: more prominent near the isthmus, they are most apparent in the proliferative phase but still visible in the secretory phase. These cells occur most often in estrogen stimulated endometrial they are smaller than secretory cells and are columnar in palisading arrangements.
-Stromal cells: in early proliferative phase, they are undifferentiated fibroblast cells which are small and ovoid. They are loosely grouped or can appear singly in the tissue. As the menstrual cycle proceeds, the stromal cells become more fibroblast like, spindled and larger. Mid cycle these cells become grouped. During pregnancy these cells become enlarged.
Some Endometrial Pathologies:
Non specific endometritis: inflammation of the endometrium from an undetermined cause. Usually a variety of factors are associated with this form of inflammation such as:
-Disruption of the cervical mucous barrier
-Intrauterine necrosis
-Interruption of regular endometrial shedding
As the inflammation become more severe the entire corpus is inflamed including the basalis, this reaction interrupts the endometrium’s normal processes.
Endometrial Hyperplasia: Abnormal proliferation of endometrial cells; it can cause the endometrium to grow outside the uterus. These cells still respond to hormonal stimuli in the body and can cause endometrial tissue build up on organs outside the uterus.
Carcinoma of the endometrium: related to endometrial hyperplasia, it is a cancer of the endometrium. Usually the carcinoma is preceeded by severe hyperplasia in young women. It typically occurs in older females and in particular females with:
-Diabetes
-Obesity
-Infertility
-Late onset menopause
It is characterized by polyp-like malignant tumors on the surface of the endometrium. These tumors cause the uterus to expand and the uterine cavity to become blocked with tumors.
References:
Aplin, John D., Fazleabas, Asgerally T., Glasser. Stanley R. and Giudice, Linda C. (Editors) (2008) The Endometrium: Molecular, cellular and Clinical Perspectives. Informa Healthcare: London.
Buckley, C.H. and Fox, H. (2002) Biopsy Pathology of the Endometrium: Second Edition. Arnold: New York.
Tae, Liang-Che (1993) Cytopathology of the Endometrium: Direct Intrauterine Sampling. American Society of Clinical Pathologists: Chicago.
Figure 1: http://buttercuppunch.files.wordpress.com/2009/04/uterus.jpg
Figure 2 : http://www.kumc.edu/instruction/medicine/anatomy/histoweb/female/small/Fem12s.JPG
Figure 3 : http://www.apsu.edu/thompsonj/Anatomy%20&%20Physiology/2020/2020%20Exam%20Reviews/Exam%205/27-14a_Uterus_1.jpg
Figure 4 : http://www.hsc.stonybrook.edu/gyn-atlas/images/DSCN3423B.jpg
Figure 5 : http://image.tutorvista.com/content/reproduction-in-animals/menstrual-cycle-phases.jpeg
The endometrium is triangular and continuous, in its upper corners, with the lumina of the fallopian tubes and the cervix at it lower point.

Figure 1: Diagram of the Female Reproductive Tract.
The endometrial lining is composed of columnar epithelial cells supported by a cellular stroma with tubular glands.
Figure 2: Endometrial Tissue Composed of Columnar Epithelia, Stroma and Tubluar Glands(Arrows).
The uterine mucosa is composed of the isthmus and corpus. The isthmus is thin mucosa and is not very responsive to hormonal stimuli; it is located between the endometrium and the endocervix in the lower portion of the uterine cavity. The corpus (endometrium) is a mucosa which is fully responsive and affected by female hormonal stumuli and their cyclic fluctuations. The corpus itself can be divided into two layers: the lower layer known as the basalis and the upper layer known as the functionalis.

Figure 3: Diagram of the Female Uterus in Order to Highlight the Isthmus and the Corpus of the Endometrium.
The basalis is the zone of relative inactivity adjacent to the myometrium of the uterus. The basalis is an area of weakly proliferative gland cells and dense spindle shaped stromal cells. It is known as the reserve cell layer and it is responsible for regenerating the endometrium after the functionalis has shed. Its thickness remains constant throughout the menstrual cycle. The functionalis is a region of proliferative glandular cells which are arranged in sheets due to the mutual adhesiveness between gland cells. Little proliferation of cells is shown in the early phases of the menstrual cycle; however as estrogen levels rise, proliferation is more apparent and in the late proliferatory phase, the epithelial layer changes to a pseudostratified or stratified columnar epithelium. Since the endometrial tissue is highly sensitive to female steroid hormone levels in the body, its composition undergoes many changes depending on a woman’s age and fertility.
Before Puberty: Estrogen and progesterone levels are low and the endometrium is atrophic showing a lack of mitotic activity in its gland it is similar to the endometrial state after menopause.
After Menopause: The endometrium is once again atrophic. The lining is thin and smooth. The glands are a single layer of flattened cuboidal cells and the stromal cells are spindled and closely packed.

Figure 4: Histological Section of an Atrophic Endometrium.
During the Reproductive Years: The endometrium is composed of:
• Glandular epithelia
• Proliferative glands
• Secretory glands
• Surface glandular epithelium
• Stoma
• Vasculature
The endometrial cells synchronously proliferate, differentiate and shed cyclically every 28 days in response to estradiol and progesterone changes in the female human body. This 28 day cycle is known as the Menstrual Cycle and is categorized into phases.
Proliferation Phase:
Days 1-14 of the cycle. This phase exhibits ovarian secreted, estradiol induced proliferation of stroma, gland cells and vasculature.
Days 1-4 mark the menstrual phase which will be discussed below.
Days 4-13: proliferation of the endometrial lining ensues. The functionalis tissues increase in thickness with an increase in gland cells, stroma and vasculature. The glands and blood vessels increase in size and number faster than the stroma, causing them to become coiled giving the arteries a characteristic spiral conformation. The stromal cells become enlarged by edema and an increase in glycogen content and pack closer together.
Secretory Phase:
Occurs after ovulation on day 14. Estradiol and progesterone secreted from the corpus luteum induce secretions and differentiation of stromal cells into predecidualized cells.
Days 14-28: secretions are noticed in the epithelial layer The endometrium is abundant with closely packed gland and enlarged glands. In late secretory phase, the stomal cells become predecidual and surround the spiral arteries. The secretions slow down and neutrophils and lymphocyte-like granulocytes infiltrate the stroma. These granulocytes are thought to release relaxin that dissolve reticular fibers found near the stromal cells just before endometrial shedding of the menstrual phase.
If implantation does not occur the menstrual phase progresses.
Menstrual Phase:
Days 1-4 Endometrial shedding takes place. Around the 28th day of the menstrual cycle, there is a sudden reduction in the thickness of the functionalis due to a decreased steroid hormone levels in the body. This causes impaired blood flow to the upper portion of the endometrium leading to ischemia, necrosis and hemorrhage of the endometrial lining. During this phase, there is an increase in leukocytes and fibrin thrombin appears in blood vessels in the area. The collapsed stroma, ruptured glands, neutrophils, granulocytes and ruptured vasculature is what is shed during this phase.

Figure 5: Diagram of Endometrial Thickness in Response to Hormonal Stimulation.
However if implantation does occur before shedding, there is a resurgence of gland secretions and exaggerated stromal decidualization where almost all endometrial stroma is converted to sheets of large decidual cells.
Cells of the Endometrium
-Ciliated glandular cells: more prominent near the isthmus, they are most apparent in the proliferative phase but still visible in the secretory phase. These cells occur most often in estrogen stimulated endometrial they are smaller than secretory cells and are columnar in palisading arrangements.
-Stromal cells: in early proliferative phase, they are undifferentiated fibroblast cells which are small and ovoid. They are loosely grouped or can appear singly in the tissue. As the menstrual cycle proceeds, the stromal cells become more fibroblast like, spindled and larger. Mid cycle these cells become grouped. During pregnancy these cells become enlarged.
Some Endometrial Pathologies:
Non specific endometritis: inflammation of the endometrium from an undetermined cause. Usually a variety of factors are associated with this form of inflammation such as:
-Disruption of the cervical mucous barrier
-Intrauterine necrosis
-Interruption of regular endometrial shedding
As the inflammation become more severe the entire corpus is inflamed including the basalis, this reaction interrupts the endometrium’s normal processes.
Endometrial Hyperplasia: Abnormal proliferation of endometrial cells; it can cause the endometrium to grow outside the uterus. These cells still respond to hormonal stimuli in the body and can cause endometrial tissue build up on organs outside the uterus.
Carcinoma of the endometrium: related to endometrial hyperplasia, it is a cancer of the endometrium. Usually the carcinoma is preceeded by severe hyperplasia in young women. It typically occurs in older females and in particular females with:
-Diabetes
-Obesity
-Infertility
-Late onset menopause
It is characterized by polyp-like malignant tumors on the surface of the endometrium. These tumors cause the uterus to expand and the uterine cavity to become blocked with tumors.
References:
Aplin, John D., Fazleabas, Asgerally T., Glasser. Stanley R. and Giudice, Linda C. (Editors) (2008) The Endometrium: Molecular, cellular and Clinical Perspectives. Informa Healthcare: London.
Buckley, C.H. and Fox, H. (2002) Biopsy Pathology of the Endometrium: Second Edition. Arnold: New York.
Tae, Liang-Che (1993) Cytopathology of the Endometrium: Direct Intrauterine Sampling. American Society of Clinical Pathologists: Chicago.
Figure 1: http://buttercuppunch.files.wordpress.com/2009/04/uterus.jpg
Figure 2 : http://www.kumc.edu/instruction/medicine/anatomy/histoweb/female/small/Fem12s.JPG
Figure 3 : http://www.apsu.edu/thompsonj/Anatomy%20&%20Physiology/2020/2020%20Exam%20Reviews/Exam%205/27-14a_Uterus_1.jpg
Figure 4 : http://www.hsc.stonybrook.edu/gyn-atlas/images/DSCN3423B.jpg
Figure 5 : http://image.tutorvista.com/content/reproduction-in-animals/menstrual-cycle-phases.jpeg
Subscribe to:
Posts (Atom)